Introduction
Allogeneic stem cell transplant (HCT) brings survival benefit to certain subgroups of acute myeloid leukemia (AML) patients, although it may not be suitable for all cases. Decision for HCT referral is based on the ELN risk grouping, which classifies pts into favorable, intermediate, and adverse risk categories. While HCT is recommended for pts in the intermediate and adverse risk, those in the favorable risk are deferred to receive HCT. However, currently, there is a lack of data directly demonstrating benefits of HCT in specific mutation groups.
Patients and Method
In this retrospective study, out of 1477 newly diagnosed AML pts 1,228 pts achieved CR1 and had undergone baseline next-generation sequencing (NGS) at diagnosis from January 1997 to January 2020. The data were collected from five different centers located in Toronto, Vancouver, and Montreal, Canada, as well as Hwasun and Seoul, South Korea. NGS was performed in each institution with each institution's standard targeted sequencing panel. To assess the effectiveness of HCT, particular attention will be given to the time from achieving complete remission 1 (CR1) to HCT. To handle immortal time bias, the Mantel-Byar method was used to depict outcomes during the follow-up period. Kaplan-Meier curves were avoided as they can introduce immortal bias, considering the period of time before treatment as part of the treatment arm. Instead, the MBT and landmark methods were employed to address this issue. Allogeneic HCT was treated as a time-dependent variable to prevent immortal bias. Gene mutations were grouped into 6 biologic pathways, including DNA methylation, chromatin modifiers, cohesion complex, activated signaling, tumor suppressor, spliceosome, and myeloid transcription factor (TF) mutations. In the multivariate (MVA) analysis, any mutations with a frequency of >5% were included as well as TP53 which has very grave prognosis. The primary objective of this study is to identify specific groups of AML pts who can derive significant benefits from receiving HCT in CR1, treating HCT as a time-dependent (td) covariate in the analysis. Statistical analyses were conducted using EZR version 1.41.
Results
The median duration of follow-up among survivors after achieving CR1 was 22.6 months (range: 1-217.9 months). Among the 593 pts, who underwent allogeneic HCT in CR1, the HCT group was composed of younger pts, with a median age of 50 years compared to 56 years in the non-HCT group (p < 0.001). Also, there were fewer pts with NPM1 mutation in the HCT group, accounting for 20.7% compared to 32.4% in the non-HCT group (p < 0.001). Apart from these characteristics, the two groups were similar in other parameters.
The 2-year overall survival (OS) was higher in the HCT group, at 66.4%, compared to 54.6% in the non-HCT group (p < 0.0001). Similarly, the 2-year RFS was higher in the HCT group, with a rate of 64.8%, compared to 52.1% in the non-HCT group (p =1.82e-15). Furthermore, HCT as a time-dependent covariate was found to be favorable (Hazard ratio [HR] 0.76, 95% C.I. [0.63-0.91]; p=0.003), as demonstrated in the Simon-Makuch plot (p = 0.003).
In the MVA, HCT as a time-dependent covariate retained its favorable impact on RFS (HR 0.67, [0.56-0.82]; p<0.0001). The MVA showed several prognostic factors besides HCT such as NPM1 and CEBPA mutations as favorable factors for RFS, being classified under the European Leukemia Network (ELN) favorable risk group. Conversely, FLT3-ITD, DNMT3A, IDH1, KIT, and TP53 mutations, monosomal karyotype, age > 60 years, and white blood count (WBC) at diagnosis > 55,600/mm^3 were associated with worse RFS.
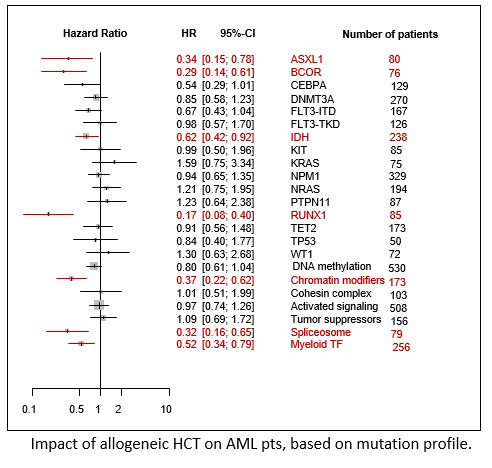
In terms of RFS, the greatest benefit of HCT was observed in the pts having mutations in ASXL1 (HR 0.34 [0.15-0.78]), BCOR (HR 0.29 [0.14-0.61]), IDH1/2 (HR 0.62 [0.42-0.92]), and RUNX1 (HR 0.17 [0.08-0.40]) but no significant benefit in those with KRAS (HR 1.59), NRAS (HR 1.21), PTPN11 (HR 1.23), and WT1 (HR 1.30). According to the biological pathway of somatic mutations, the greatest benefit of HCT in terms of RFS was observed in pts with mutations in the chromatin modifier (HR 0.37 [0.22-0.62]), spliceosome (HR 0.32 [0.16-0.65]), and myeloid TF pathway (HR 0.52 [0.34-0.79]).
Conclusion
Allogeneic HCT exerts a greater benefit on RFS in AML pts with mutations in the chromatin modifier, spliceosome, and myeloid TF pathways. Further study is warranted to replicate this finding.
Disclosures
Bergeron:Amgen: Honoraria; Abbvie: Honoraria; Gilead: Honoraria; BMS: Honoraria; Jazz: Honoraria; Taiho: Honoraria; Pfizer: Honoraria. Sanford:AbbVie: Honoraria; Astellas: Honoraria. Schuh:Amgen: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Glycomimetics: Research Funding; Astellas: Honoraria, Research Funding; Kite/Gilead: Research Funding; Pfizer: Consultancy, Honoraria; Servier: Honoraria, Research Funding; Teva: Consultancy, Honoraria. Kim:Paladin: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Novartis: Consultancy, Honoraria, Research Funding.